Every breast cancer survivor lives with the same silent fear:
“What if it comes back?”
Advanced Clinical Insights, Emerging Technologies & Patient Impact
Breast cancer remains one of the most prevalent cancers worldwide, and recurrence continues to be a major concern for survivors. Even after successful treatment, microscopic disease can persist and later re-emerge. Traditional surveillance relies heavily on imaging and clinical exams, but these methods often detect recurrence only after a measurable tumor develops.
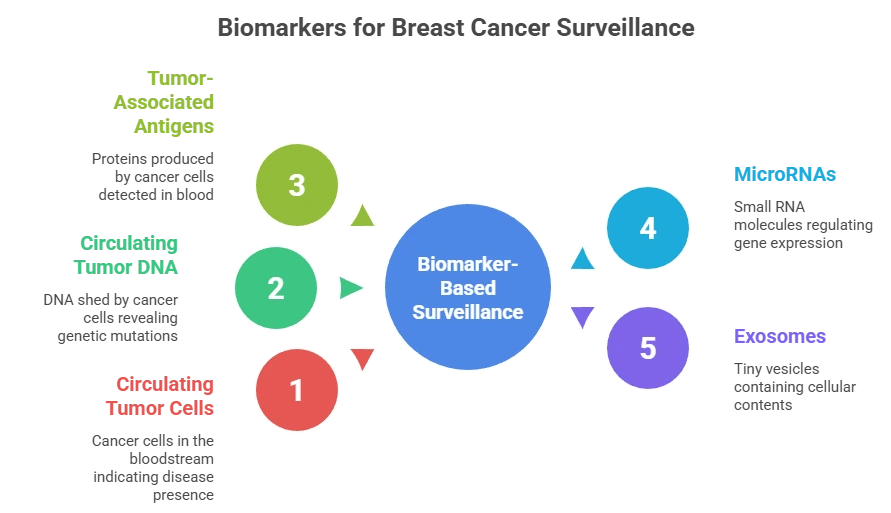
Biomarkers are transforming this paradigm. By detecting molecular changes in blood or tissue, biomarkers can reveal recurrence months before symptoms or scans—allowing earlier intervention, personalized treatment, and improved outcomes.

Power of Prevention
powerofprevention@outlook.com
This comprehensive guide explores the science, clinical evidence, innovations, limitations, and future of biomarker-based breast cancer surveillance.
Understanding Biomarkers in Cancer Monitoring
Biomarkers are measurable biological indicators found in blood, tissue, or other fluids that reflect disease activity.
Key Biomarker Types in Breast Cancer
| Biomarker | What it Detects | Clinical Role |
|---|---|---|
| Circulating Tumor Cells (CTCs) | Whole cancer cells in blood | Prognosis, treatment response |
| Circulating Tumor DNA (ctDNA) | Tumor-specific DNA fragments | Early recurrence, MRD detection |
| Protein Markers (CA 15-3, CEA) | Tumor-associated proteins | Disease monitoring trends |
| MicroRNAs (miRNAs) | Regulatory RNA from cancer cells | Emerging recurrence markers |
| Exosomes | Tumor-derived vesicles | Multi-marker profiling |
These biomarkers provide real-time molecular insight into cancer behavior, often before imaging reveals disease.
Role of Biomarkers in Detecting Recurrence
Breast cancer recurrence may be:
- Local (breast or lymph nodes)
- Distant/metastatic (bone, liver, lung, brain)
Traditional imaging may miss early molecular relapse. In contrast:
ctDNA can predict recurrence up to 10 months earlier than imaging
(Garcia-Murillas et al., Science Translational Medicine, 2015)
This allows oncologists to:
- Start treatment earlier
- Change therapy before resistance develops
- Reduce metastatic spread
Specific Biomarkers in Clinical Use
1. CA 15-3 & CEA
- Moderate sensitivity (~70%)
- Best used as trend markers
- Elevated in metastatic disease
2. Circulating Tumor DNA (ctDNA)
- Highly tumor-specific
- Tracks genetic mutations
- Detects minimal residual disease (MRD)
3. Circulating Tumor Cells (CTCs)
- 5 CTCs / 7.5 mL blood = poorer survival
- Predicts recurrence risk
4. Emerging Markers
- miR-21, miR-155 (recurrence predictors)
- Exosomal proteins & RNA
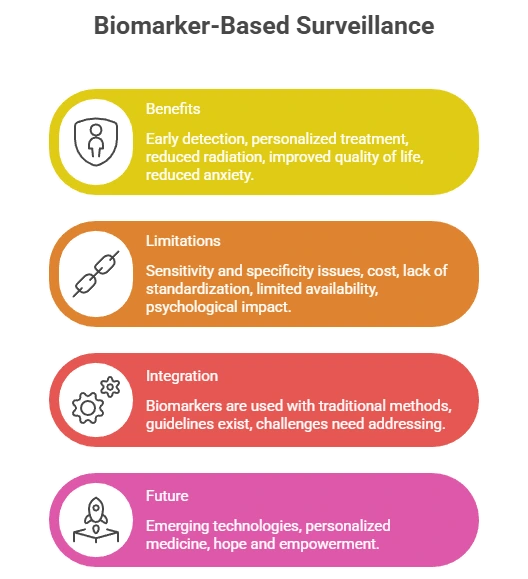
Integration into Clinical Practice
ASCO & ESMO Guidance
- CA 15-3 & CEA: adjunct surveillance for Stage II–III
- ctDNA: recommended for high-risk patients
- Not standalone—used with imaging
Clinical Applications
- Early relapse detection
- Therapy resistance monitoring
- Post-surgery MRD tracking
- Personalized surveillance schedules
Emerging Technologies
Liquid Biopsy Platforms
- Next-Generation Sequencing (NGS)
Detects mutations at <0.01% levels - Droplet Digital PCR (ddPCR)
Ultra-sensitive quantification
Artificial Intelligence
AI models combining ctDNA, CTCs, and clinical data predict recurrence with >90% accuracy in pilot studies.
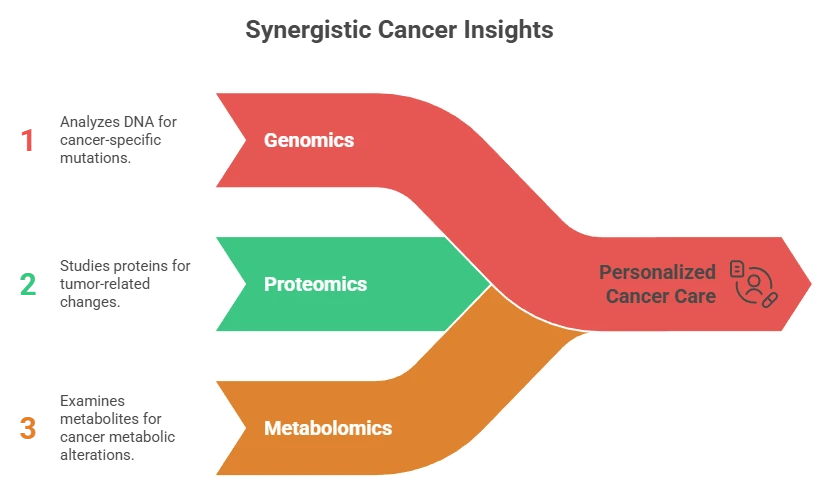
Multi-Omics Monitoring
Combines:
- Genomics (ctDNA)
- Proteomics (exosomes)
- Metabolomics
Improves predictive accuracy by 20–30%.
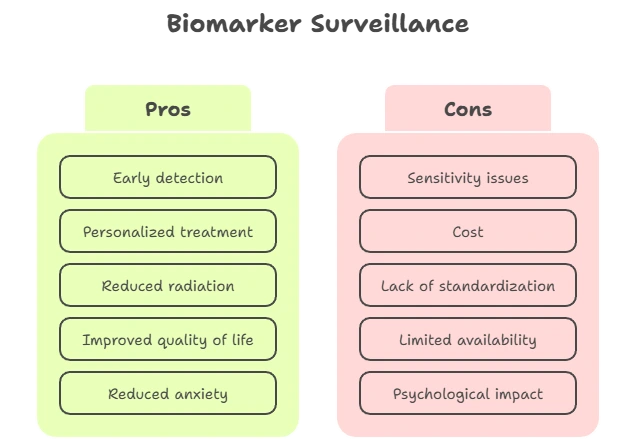
Challenges & Ethical Issues
| Issue | Impact | Mitigation |
|---|---|---|
| False positives | Anxiety, overtreatment | Serial testing + imaging |
| Lab variability | Inconsistent results | Standardization protocols |
| Cost & access | Global disparity | Low-cost liquid biopsy programs |
| Genetic privacy | Data misuse | HIPAA, GDPR, GINA protections |
Patient & Global Impact
- Reduces anxiety through early reassurance
- Enables proactive care
- Limited access in low-resource regions
- Global programs are expanding affordable testing
Future Outlook
- Subtype-specific biomarker panels (TNBC, HER2+)
- AI-driven personalized surveillance
- Home blood-based monitoring
- Multi-omics recurrence forecasting
Conclusion
Biomarkers are redefining how breast cancer recurrence is detected and managed. From early molecular warning signs to personalized treatment strategies, they offer survivors a powerful tool for long-term health.
As technology advances and accessibility improves, biomarker-guided care will become central to precision oncology.
Medical Disclaimer
This content is for educational purposes only and not a substitute for professional medical advice. Always consult a qualified healthcare provider.
Frequently Asked Questions (FAQs)
Biomarkers for Breast Cancer Recurrence Monitoring
1. What are biomarkers in breast cancer?
Biomarkers are measurable biological substances found in blood, tissue, or other body fluids that provide information about cancer activity. In breast cancer, biomarkers help detect disease recurrence, monitor treatment response, and guide personalized therapy.
2. Can biomarkers detect breast cancer recurrence earlier than scans?Yes. Advanced biomarkers such as circulating tumor DNA (ctDNA) can detect molecular signs of recurrence months before imaging or symptoms appear, allowing earlier treatment.
3. What is ctDNA and why is it important?
ctDNA (circulating tumor DNA) consists of tiny fragments of genetic material released by cancer cells into the bloodstream. It is highly specific to the tumor and helps detect minimal residual disease and early relapse.
4. Are CA 15-3 and CEA reliable for monitoring recurrence?
CA 15-3 and CEA are useful for tracking disease trends, but they are not cancer-specific. Rising levels over time may indicate recurrence and usually prompt further imaging or testing.
5. What are circulating tumor cells (CTCs)?
CTCs are whole cancer cells that break away from the tumor and circulate in the blood. Higher CTC counts are associated with increased risk of recurrence and poorer prognosis.
6. How often should biomarker tests be done?
Testing frequency depends on cancer stage, risk profile, and treatment type. High-risk patients may undergo testing every 3–6 months as part of follow-up care.
7. Are biomarker tests painful or invasive?
No. Most biomarker tests require only a simple blood draw, making them far less invasive than biopsies or repeated imaging scans.
8. Can biomarkers replace mammograms or MRIs?
No. Biomarkers complement imaging but do not replace it. Imaging shows tumor location, while biomarkers provide molecular-level detection.
9. Do all breast cancer patients benefit from biomarker testing?
Not all patients show detectable biomarker levels. They are most beneficial in high-risk, advanced-stage, or previously treated patients.
10. What is minimal residual disease (MRD)?
MRD refers to microscopic cancer cells remaining after treatment. Biomarkers like ctDNA can detect MRD long before clinical recurrence occurs.
11. Can biomarkers guide treatment changes?
Yes. Rising ctDNA or CTC levels can signal therapy resistance, prompting oncologists to change or intensify treatment.
12. Are biomarker tests covered by insurance?
Coverage varies by country and insurer. Many ctDNA tests are still considered advanced diagnostics and may require special approval.
13. Are there risks of false positives?
Yes. Some biomarkers may rise due to non-cancer conditions. Results are always interpreted with imaging and clinical findings.
14. What new biomarkers are being developed?
MicroRNAs, exosomes, and multi-omics liquid biopsy panels are emerging tools showing promise for early recurrence detection.
15. Should breast cancer survivors ask about biomarker testing?
Yes. Survivors—especially those with high-risk or advanced disease—should discuss biomarker monitoring with their oncologist.
References
- Living Beyond Breast Cancer (LBBC).
Circulating tumor DNA (ctDNA) in breast cancer monitoring. Accessed February 3, 2026.
https://www.lbbc.org/about-breast-cancer/testing/biomarker/ctdna - Chan KCA, et al.
Clinical utility of circulating tumor DNA for early detection of breast cancer recurrence. NPJ Precision Oncology. 2025;9:76. - Coombes RC, et al.
Personalized detection of molecular residual disease in breast cancer using ctDNA. Translational Breast Cancer Research. 2021;2:28. - Duffy MJ, et al.
Tumor markers in breast cancer: CA 15-3 and CEA in follow-up. Clinical Biochemistry. 2020;82:1–7. - Guardian Health Desk.
Predictive blood test hailed as breakthrough for early breast cancer relapse detection. The Guardian. June 2, 2024.
Dr. Mohammed Abdul Azeem Siddiqui, MBBS, M.Tech (Biomedical Engineering – VIT, Vellore)
Registered Medical Practitioner – Reg. No. 39739
Physician • Clinical Engineer • Preventive Diagnostics Specialist
Dr. Mohammed Abdul Azeem Siddiqui is a physician–engineer with over 30 years of dedicated clinical and biomedical engineering experience, committed to transforming modern healthcare from late-stage disease treatment to early detection, preventive intelligence, and affordable medical care.
He holds an MBBS degree in Medicine and an M.Tech in Biomedical Engineering from VIT University, Vellore, equipping him with rare dual expertise in clinical medicine, laboratory diagnostics, and medical device engineering. This allows him to translate complex laboratory data into precise, actionable preventive strategies.
Clinical Mission
Dr. Siddiqui’s professional mission centers on three core pillars:
Early Disease Detection
Identifying hidden biomarker abnormalities that signal chronic disease years before symptoms appear — reducing complications, hospitalizations, and long-term disability.
Preventive Healthcare
Guiding individuals and families toward longer, healthier lives through structured screenings, lifestyle intervention frameworks, and predictive diagnostic interpretation.
Affordable Evidence-Based Treatment
Delivering cost-effective, scientifically validated care accessible to people from all socioeconomic backgrounds.
Clinical & Technical Expertise
Across three decades of continuous practice, Dr. Siddiqui has worked extensively with:
Advanced laboratory analyzers and automation platforms
• Cardiac, metabolic, renal, hepatic, endocrine, and inflammatory biomarker systems
• Preventive screening and early organ damage detection frameworks
• Clinical escalation pathways and diagnostic decision-support models
• Medical device validation, calibration, compliance, and patient safety standards
He is recognized for identifying subclinical biomarker shifts that predict cardiovascular disease, diabetes, fatty liver, kidney disease, autoimmune inflammation, neurodegeneration, and accelerated biological aging long before conventional diagnosis.
Role at IntelliNewz
At IntelliNewz, Dr. Siddiqui serves as Founder, Chief Medical Editor, and Lead Clinical Validator. Every article published is:
Evidence-based
• Clinically verified
• Technology-grounded
• Free from commercial bias
• Designed for real-world patient and physician decision-making
Through his writing, Dr. Siddiqui shares practical health intelligence, early warning signs, and preventive strategies that readers can trust — grounded in decades of frontline medical practice.
Contact:
powerofprevention@outlook.com
📌 Disclaimer: The content on IntelliNewz is intended for educational purposes only and does not replace personalized medical consultation. For individual health concerns, please consult your physician.



