Immune Aging Starts in Your 30s (Not at Retirement)
For decades, the concept of a declining immune system—immunosenescence—was framed as a problem for the elderly, a consequence of reaching retirement age.
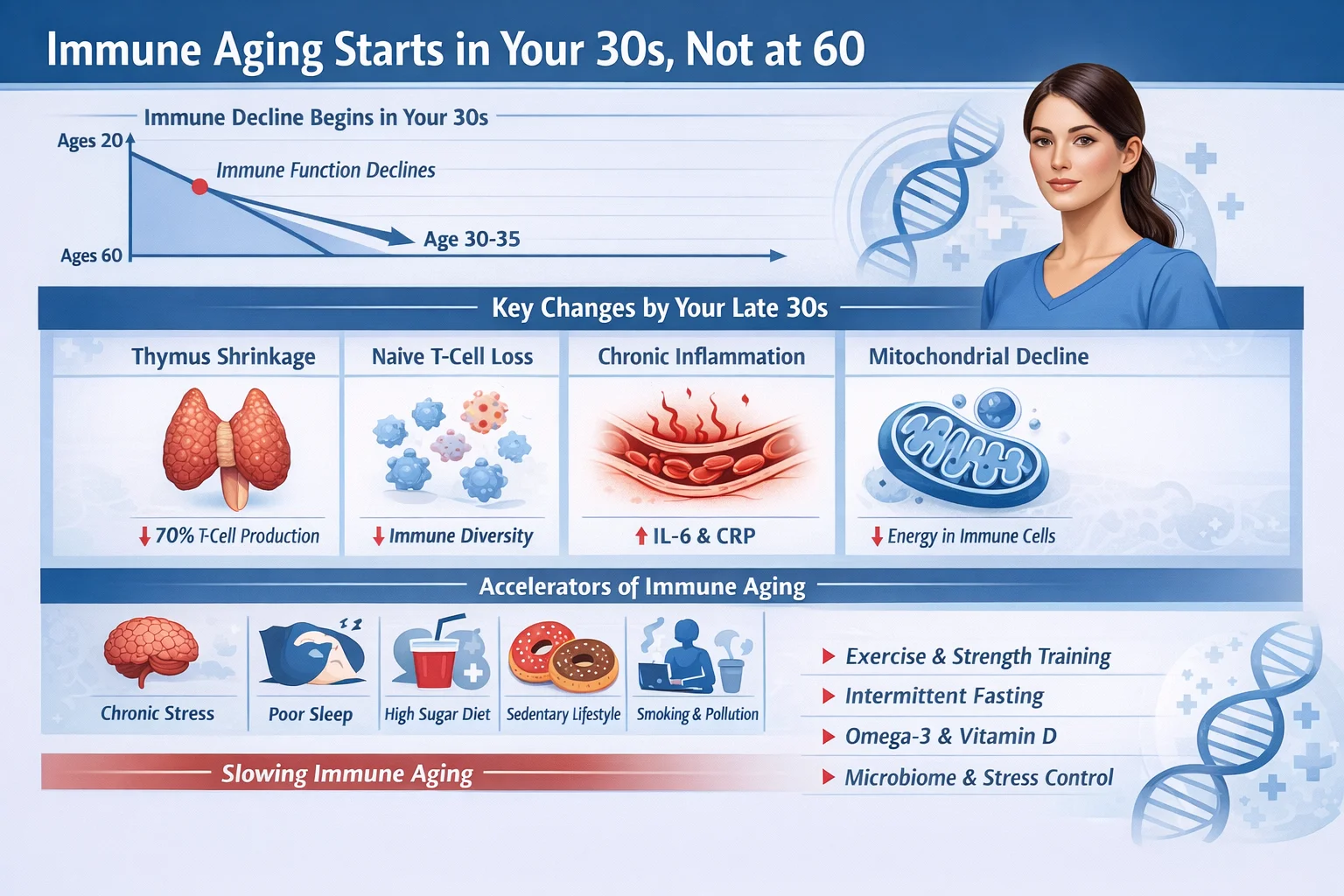
We imagined that for most of our lives, our immunological defenses were on a long, flat plateau, only to fall off a cliff in our golden years. However, a wave of modern immunological research has shattered this paradigm. Groundbreaking studies now show that the architecture of our immune system begins a significant structural decline much earlier than previously thought—not at 65, but as early as our 30s .
This isn’t about feeling a little more tired after a night out; it is a fundamental biological remodeling that starts in the third decade of life, altering the very composition of our defenses and setting the stage for health outcomes decades later.
The 30-Year-Old Threshold: A Structural Shift
The idea that immune decline is a linear, late-life event has been replaced by a more complex and nuanced understanding. Researchers have identified critical junctures where the immune system undergoes rapid, non-linear changes.
While a significant “wave” of aging occurs around 60, the first major ripple is detected in the late 30s to early 40s .
This early phase of aging is not a dramatic functional collapse but a subtle yet profound shift in the production and balance of our immune cells.
The primary drivers of this shift are two-fold: the involution of the thymus and the changing nature of our hematopoietic (blood-forming) stem cells.
1. The Fading Thymus: The thymus, a small organ located behind the breastbone, is the boot camp for our T cells, a critical part of the adaptive immune system responsible for recognizing and fighting new, never-before-seen pathogens.
While thymic shrinkage (involution) begins in childhood, it reaches a critical point of near-complete atrophy around age 30 . This drastically reduces the output of new, or “naïve,” T cells. With the factory shutting down, the body must rely on an existing pool of T cells that are already “memory” cells—trained to fight old battles but ill-equipped for new threats .
2. Stem Cell Exhaustion: The problem is compounded at the source. Hematopoietic stem cells (HSCs) in our bone marrow, which give rise to all immune cells, begin to accumulate DNA mutations and experience telomere shortening with age . By our 30s, the pool of progenitor cells for new B and T cells starts to dwindle.
Furthermore, the bone marrow environment itself changes, becoming fatty and less supportive of lymphopoiesis (the creation of lymphoid cells). This creates a “myeloid bias,” meaning the body starts producing more innate immune cells (the generalists) and fewer adaptive immune cells (the specialists), skewing the entire system’s response profile .
The Molecular Clock and the “Immune Age”
Recent multi-omic studies have provided unprecedented detail into these changes. A landmark study published in Nature used single-cell RNA sequencing to profile the immune systems of healthy adults from age 25 to 90, creating a dataset of over 16 million cells.
This research confirmed that T cells undergo the most pronounced molecular reprogramming with age, changes that are distinct from those caused by inflammation or chronic infections like cytomegalovirus .
This study also introduced the concept of an “RNA Age Metric” (RAM)—a composite score of gene activity that reflects one’s biological “immune age.” This metric rises in a non-linear fashion, beginning well before old age, and suggests that we can now measure the acceleration or deceleration of this process .
Similarly, research from Shanghai Jiao Tong University mapped immune cell evolution across the entire human lifespan, finding that while some T cell subsets peak in childhood and adolescence, CD8+ naïve T cells decline continuously from adolescence onward, a trajectory that begins its downward arc in our 20s and 30s .
What Declines, and What Rises?
So, what does immune aging in your 30s actually look like under the microscope?
- Naïve T Cells Decrease: The number of “virgin” T cells, ready to tackle a novel coronavirus or a mutated flu strain, plummets .
- Memory T Cells Accumulate: The immune system becomes crowded with older, less flexible memory cells. This is why older individuals are often better at fighting off strains of flu they’ve seen before but struggle with new variants .
- CD4+/CD8+ Ratio Shifts: The delicate balance between helper T cells (CD4+) and killer T cells (CD8+) begins to change, a hallmark of an aging system .
- Inflamm-Aging Begins: Although systemic inflammation may not be overtly high until later years, the seeds of “inflamm-aging”—a chronic, low-grade inflammation—are sown. As senescent cells (aged, dysfunctional cells) accumulate, they secrete inflammatory signals that stress surrounding healthy cells, creating a positive feedback loop of decline .
Not Just Time: Lifestyle as the Accelerator
The discovery that immune aging begins in our 30s is not a reason for despair but a call to action. This new timeline reveals a critical window of opportunity. Crucially, this process is not set in stone by chronological age alone; it is heavily influenced by “biological age,” which is modifiable.
A major study published in 2025, drawing from the National Longitudinal Study of Adolescent to Adult Health, examined over 4,400 adults aged 33-44 and found a direct link between social health and immune aging. It revealed that a higher number of close friends and frequent social contact were associated with a less aged immune profile, specifically a healthier ratio of naïve to memory CD4+ T cells .
This suggests that psychosocial factors are not just linked to mental health, but are biologically embedded in our immune system’s aging process by midlife.
Other accelerants of immune aging include chronic stress, poor nutrition, sleep deprivation, and environmental toxins . These factors can push the immune system into a state of premature “exhaustion,” accelerating the timeline of decline.
Conclusion: A New Perspective on Health
The narrative has officially changed. Immunosenescence is not a distant consequence of retirement, but a gradual process that begins its molecular and cellular shift when we are in our 30s.
The near-complete atrophy of the thymus by this age and the subsequent decline in naïve T cell production mark the true onset of immune aging .
This reframing empowers us. It shifts the focus from late-stage intervention to early-life preservation. It suggests that the lifestyle choices, stress management, and social connections we cultivate in our 30s and 40s are not just for our present well-being, but are direct investments in the resilience of our future selves.
The battle for a robust immune system in old age is, it turns out, first fought in the decades of early midlife.
1. Can food really strengthen the immune system?
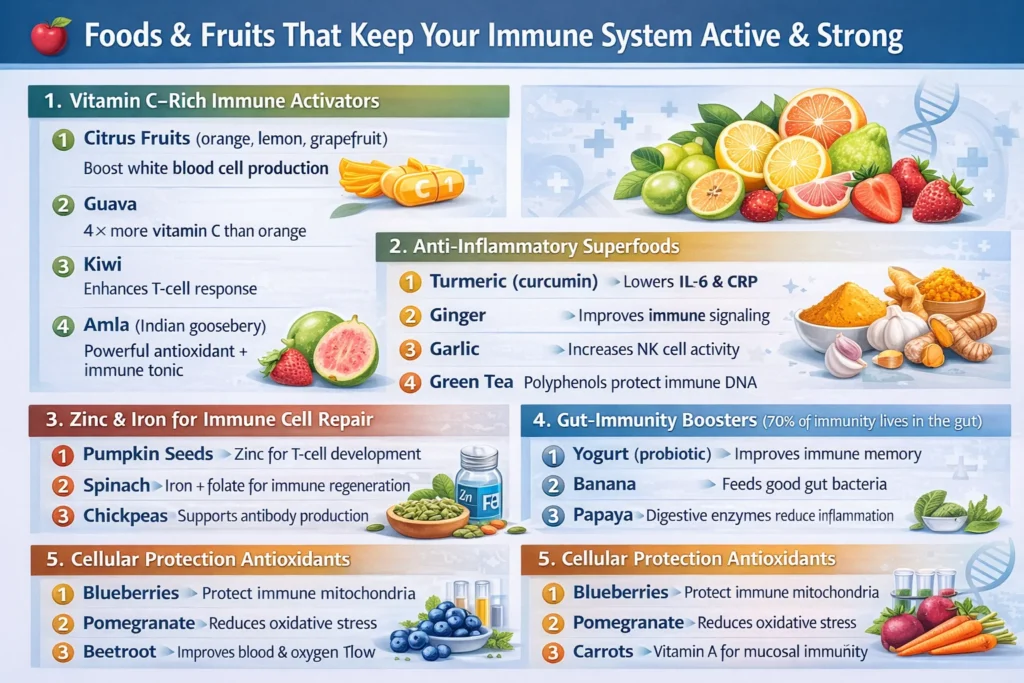
Yes. Specific nutrients like vitamin C, zinc, vitamin D, antioxidants, and probiotics directly support immune cell production, signaling, and repair. A nutrient-dense diet improves white blood cell activity and reduces chronic inflammation.
2. Which fruit is best for boosting immunity fast?
Guava, amla, kiwi, and citrus fruits are among the best. Guava alone contains 4× more vitamin C than oranges, helping the body quickly activate immune defenses.
3. How long does it take for diet to improve immunity?
Initial improvements in inflammation and energy can appear within 2–4 weeks, while stronger immune resilience develops over 8–12 weeks with consistent nutrition.
4. What foods weaken the immune system?
Ultra-processed foods, sugary drinks, refined carbs, fried foods, and excess alcohol increase inflammation and suppress immune cell function.
5. Is immunity really connected to gut health?
Yes. Over 70% of immune cells live in the gut. Probiotic and fiber-rich foods help regulate immune responses and reduce infections by strengthening the gut–immune axis.

Dr. Mohammed Abdul Azeem Siddiqui, MBBS, M.Tech (Biomedical Engineering – VIT, Vellore)
Registered Medical Practitioner – Reg. No. 39739
Physician • Clinical Engineer • Preventive Diagnostics Specialist
Dr. Mohammed Abdul Azeem Siddiqui is a physician–engineer with over 30 years of dedicated clinical and biomedical engineering experience, committed to transforming modern healthcare from late-stage disease treatment to early detection, preventive intelligence, and affordable medical care.
He holds an MBBS degree in Medicine and an M.Tech in Biomedical Engineering from VIT University, Vellore, equipping him with rare dual expertise in clinical medicine, laboratory diagnostics, and medical device engineering. This allows him to translate complex laboratory data into precise, actionable preventive strategies.
Clinical Mission
Dr. Siddiqui’s professional mission centers on three core pillars:
Early Disease Detection
Identifying hidden biomarker abnormalities that signal chronic disease years before symptoms appear — reducing complications, hospitalizations, and long-term disability.
Preventive Healthcare
Guiding individuals and families toward longer, healthier lives through structured screenings, lifestyle intervention frameworks, and predictive diagnostic interpretation.
Affordable Evidence-Based Treatment
Delivering cost-effective, scientifically validated care accessible to people from all socioeconomic backgrounds.
Clinical & Technical Expertise
Across three decades of continuous practice, Dr. Siddiqui has worked extensively with:
Advanced laboratory analyzers and automation platforms
• Cardiac, metabolic, renal, hepatic, endocrine, and inflammatory biomarker systems
• Preventive screening and early organ damage detection frameworks
• Clinical escalation pathways and diagnostic decision-support models
• Medical device validation, calibration, compliance, and patient safety standards
He is recognized for identifying subclinical biomarker shifts that predict cardiovascular disease, diabetes, fatty liver, kidney disease, autoimmune inflammation, neurodegeneration, and accelerated biological aging long before conventional diagnosis.
Role at IntelliNewz
At IntelliNewz, Dr. Siddiqui serves as Founder, Chief Medical Editor, and Lead Clinical Validator. Every article published is:
Evidence-based
• Clinically verified
• Technology-grounded
• Free from commercial bias
• Designed for real-world patient and physician decision-making
Through his writing, Dr. Siddiqui shares practical health intelligence, early warning signs, and preventive strategies that readers can trust — grounded in decades of frontline medical practice.
Contact:
powerofprevention@outlook.com
📌 Disclaimer: The content on IntelliNewz is intended for educational purposes only and does not replace personalized medical consultation. For individual health concerns, please consult your physician.