Cervical cancer remains a significant health concern for women in the United States. Early detection is crucial, as precancerous changes can be treated long before invasive cancer develops. While traditional screening methods like Pap smears and HPV DNA tests have helped reduce mortality, a new diagnostic technology is poised to revolutionize cervical cancer prevention: the CRISPR test for cervical cancer.
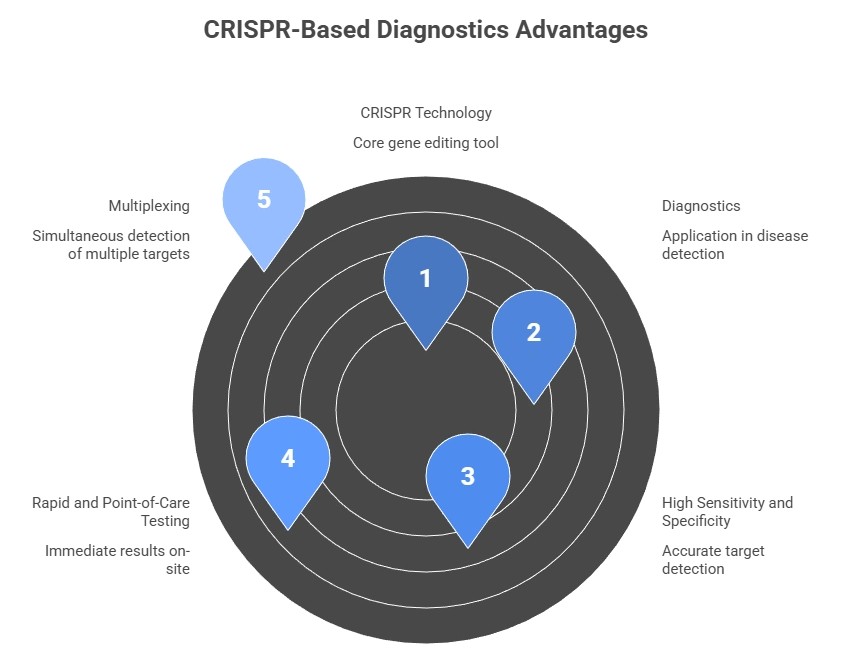
Welcome to IntelliNewz – The Power of PreventionWhat Is a CRISPR Test for Cervical Cancer?CRISPR, originally a gene-editing technology, has evolved into a powerful tool for molecular diagnostics. CRISPR-based assays such as DETECTR and SHERLOCK use enzymes like Cas12a or Cas13 to detect specific DNA or RNA sequences from high-risk human papillomavirus (HPV) strains—the main cause of cervical cancer — and cancer-related biomarkers.
Unlike traditional Pap smears, which detect abnormal cells, or HPV DNA tests, which detect viral DNA, CRISPR tests identify both viral and genetic markers with high sensitivity, speed, and precision, making early detection more accessible and accurate.
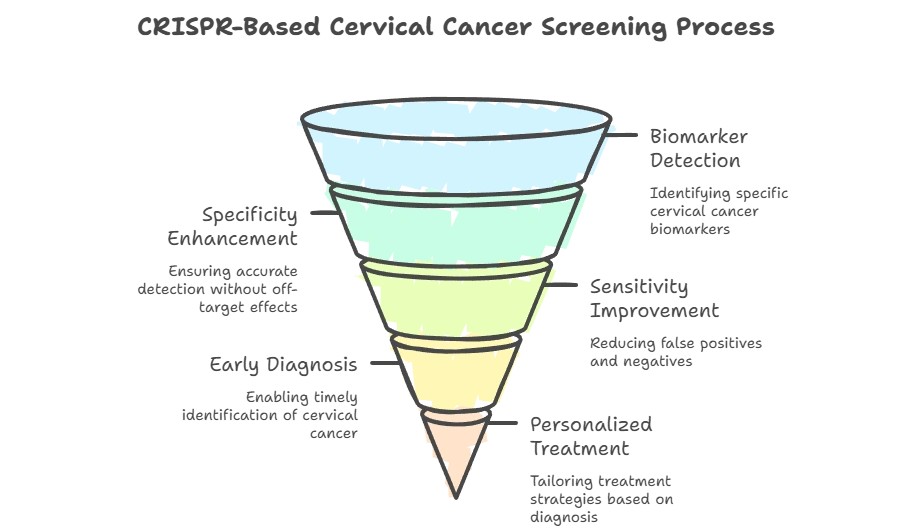
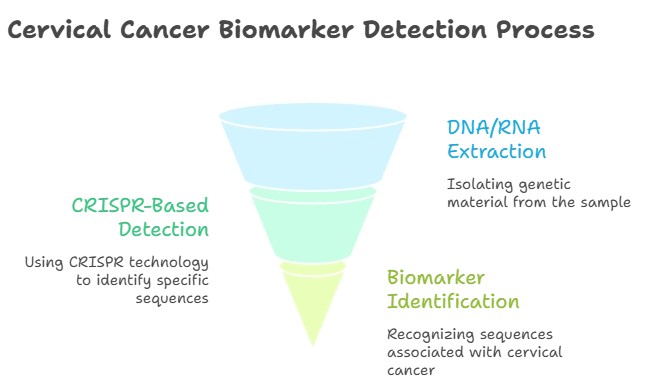
How CRISPR Tests Detect Cervical Cancer
CRISPR-based cervical cancer tests follow a simple yet advanced process:
Sample Collection
A small cervical swab, vaginal self-sample, urine, or blood sample is collected.
Amplification
Genetic material from high-risk HPV types (like HPV16/18) or cancer biomarkers is amplified using isothermal techniques such as RPA or LAMP.
CRISPR Detection
The CRISPR enzyme binds to specific DNA or RNA sequences, triggering a fluorescent or colorimetric signal if the target is present.
Results
The signal is detected in under two hours, enabling rapid point-of-care or at-home testing.
Key Benefits:
High Sensitivity and Specificity: Detects minute viral or cancer signals missed by Pap smears.
Fast Results: From hours instead of days.
Precision Screening: Identifies HPV subtypes and early cancer biomarkers.
Cost-Effective: Pilot tests cost less than $10.
Portable: Ideal for low-resource areas.
CRISPR vs Traditional Screening Methods
| Screening Method | Sensitivity | Time | Cost | Invasiveness |
| CRISPR Test | 90–97% | 1–2 hours | <$10 | Non-invasive |
| Pap Smear | 60–80% | Days | $20–50 | Mild |
| HPV DNA Test | ~90% | Hours | $50–100 | Mild |
| Colposcopy/Biopsy | 95%+ | Days | $200–500 | Moderate |
CRISPR tests outperform traditional methods in speed, affordability, and early detection capability, making them especially useful in underserved communities.
Recent Research and Developments
High-Risk HPV Detection: A 2023 study in Science Translational Medicine showed a CRISPR-Cas12a assay detecting HPV16/18 with 97% sensitivity and 100% specificity.
Cancer Biomarker Screening: CRISPR assays now detect E6/E7 oncogenes and methylation changes in genes like p16INK4a.
Point-of-Care Applications: Pilot programs in Kenya and India used portable CRISPR devices to screen women, increasing coverage and reducing unnecessary biopsies by 40–50%.
AI Integration: CRISPR outputs combined with AI models can predict cancer progression with over 90% accuracy, enabling precision screening.
Global and U.S. Health Impact
Cervical cancer affects 600,000 women worldwide, with most deaths in low-resource regions due to lack of screening. CRISPR tests could:
Increase Screening Coverage: Portable devices enable home or mobile clinic testing.
Reduce Healthcare Costs: Shifting from late-stage treatment to early detection saves billions.
Support Vaccine Monitoring: CRISPR can track HPV strains not covered by current vaccines.
Improve Equity: Women in rural or underserved areas gain access to high-quality screening.
In the U.S., CRISPR tests could complement existing screening programs, improve early detection, and help prevent cancer progression.
Challenges and Considerations
While promising, CRISPR tests face several hurdles:
Regulatory Approval: FDA approval for primary cervical cancer screening is still pending.
Training and Implementation: Requires skilled personnel for accurate use in clinics.
False Positives: Detects transient HPV infections that may not lead to cancer.
Data Privacy: Handling genetic information must comply with HIPAA regulations.
Cost and Accessibility: Initial device costs range from $500–$2000, though per-test cost remains low.
The Future of CRISPR in Cervical Cancer Screening
CRISPR-based diagnostics are poised to transform cervical cancer prevention:
Rapid, low-cost, point-of-care testing for early detection
Integration with AI for automated interpretation
Multiplex detection of HPV subtypes, cancer stages, and immune markers
Therapeutic applications targeting viral oncogenes in the future
By 2030, these technologies could help the U.S. and the world move closer to eliminating cervical cancer, aligning with WHO’s cervical cancer elimination goals.
Frequently Asked Questions
1. Can CRISPR tests replace Pap smears?
Not yet. They are a complementary tool for early HPV and biomarker detection.
2. How fast are results?
Typically 30 minutes to 2 hours, depending on the device.
3. Are CRISPR tests available at home?
Portable devices are in trials. Home-based self-collection is possible with professional oversight.
4. Are these tests FDA-approved?
Not for primary screening. Approval is expected in the next few years based on ongoing clinical trials.
Conclusion
CRISPR tests for cervical cancer represent a new frontier in early detection, combining speed, accuracy, and accessibility. For women in the U.S., integrating CRISPR diagnostics with traditional screening methods could save lives, reduce unnecessary procedures, and expand preventive care access — especially in underserved areas.
As technology advances, CRISPR is not just a test — it is a game-changer for women’s health, offering hope for a future where cervical cancer becomes preventable and treatable at its earliest stages.
Dr. Mohammed Abdul Azeem Siddiqui, MBBS, M.Tech (Biomedical Engineering – VIT, Vellore)
Registered Medical Practitioner – Reg. No. 39739
Physician • Clinical Engineer • Preventive Diagnostics Specialist
Dr. Mohammed Abdul Azeem Siddiqui is a physician–engineer with over 30 years of dedicated clinical and biomedical engineering experience, committed to transforming modern healthcare from late-stage disease treatment to early detection, preventive intelligence, and affordable medical care.
He holds an MBBS degree in Medicine and an M.Tech in Biomedical Engineering from VIT University, Vellore, equipping him with rare dual expertise in clinical medicine, laboratory diagnostics, and medical device engineering. This allows him to translate complex laboratory data into precise, actionable preventive strategies.
Clinical Mission
Dr. Siddiqui’s professional mission centers on three core pillars:
Early Disease Detection
Identifying hidden biomarker abnormalities that signal chronic disease years before symptoms appear — reducing complications, hospitalizations, and long-term disability.
Preventive Healthcare
Guiding individuals and families toward longer, healthier lives through structured screenings, lifestyle intervention frameworks, and predictive diagnostic interpretation.
Affordable Evidence-Based Treatment
Delivering cost-effective, scientifically validated care accessible to people from all socioeconomic backgrounds.
Clinical & Technical Expertise
Across three decades of continuous practice, Dr. Siddiqui has worked extensively with:
Advanced laboratory analyzers and automation platforms
• Cardiac, metabolic, renal, hepatic, endocrine, and inflammatory biomarker systems
• Preventive screening and early organ damage detection frameworks
• Clinical escalation pathways and diagnostic decision-support models
• Medical device validation, calibration, compliance, and patient safety standards
He is recognized for identifying subclinical biomarker shifts that predict cardiovascular disease, diabetes, fatty liver, kidney disease, autoimmune inflammation, neurodegeneration, and accelerated biological aging long before conventional diagnosis.
Role at IntelliNewz
At IntelliNewz, Dr. Siddiqui serves as Founder, Chief Medical Editor, and Lead Clinical Validator. Every article published is:
Evidence-based
• Clinically verified
• Technology-grounded
• Free from commercial bias
• Designed for real-world patient and physician decision-making
Through his writing, Dr. Siddiqui shares practical health intelligence, early warning signs, and preventive strategies that readers can trust — grounded in decades of frontline medical practice.
Contact:
powerofprevention@outlook.com
📌 Disclaimer: The content on IntelliNewz is intended for educational purposes only and does not replace personalized medical consultation. For individual health concerns, please consult your physician.