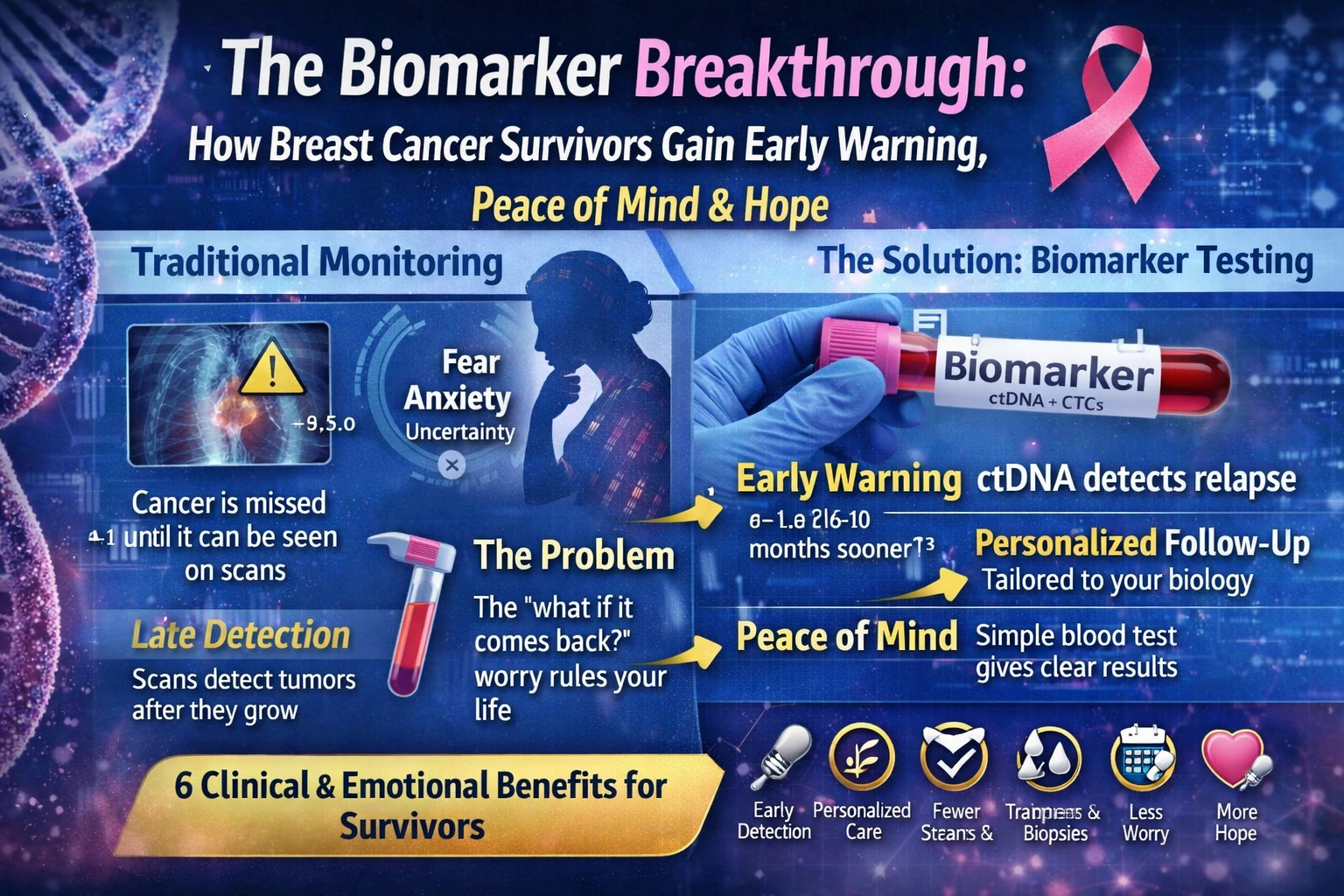
For the millions of women navigating life after a breast cancer diagnosis, the end of active treatment often marks the beginning of a new and equally challenging journey: survivorship. While the conclusion of surgery, chemotherapy, and radiation is undoubtedly a cause for celebration, it ushers in a period frequently overshadowed by a persistent and profound fear—the possibility of recurrence.

Every ache, every pain, every routine follow-up appointment can become a source of anxiety, a phenomenon often described as the “Scanxiety” that haunts the survivorship experience . However, a revolutionary shift is underway in post-treatment care.
The emergence of advanced biomarker technology, particularly circulating tumor DNA (ctDNA) testing, is transforming the landscape of breast cancer surveillance.
No longer a distant promise of the future, these breakthroughs are providing survivors with unprecedented early warning systems, tangible peace of mind, and a renewed sense of hope.
This article explores the science behind this biomarker breakthrough, examining how ctDNA testing is reshaping our understanding of remission, offering a new kind of emotional reassurance to patients, and paving the way for a future where cancer recurrence can be caught at its most treatable moment.
The Evolving Science of Breast Cancer Biomarkers
To fully appreciate the breakthrough that ctDNA represents, it is essential to understand the context of biomarkers in breast cancer. For decades, the clinical management of breast cancer has been guided by a set of “classic” biomarkers. These include the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) .
The presence or absence of these proteins on the surface of tumor cells determines the molecular subtype of the cancer and dictates the course of treatment, such as the use of hormonal therapies for ER-positive cancers or targeted therapies like trastuzumab for HER2-positive cancers.
The proliferation marker Ki-67 has also been used to gauge how quickly cancer cells are growing .
While these traditional biomarkers remain the “cornerstone” of breast cancer characterization and treatment planning, they have significant limitations . They are primarily derived from the initial tumor biopsy, providing a static snapshot of the cancer’s biology at a single point in time.
They do not capture the dynamic evolution of a tumor or the presence of microscopic residual disease after treatment . This is where the “emerging biomarkers” detailed in recent scientific literature come into play. Researchers have been investigating a host of new molecular markers, including mutations in genes like TP53, various types of non-coding RNAs, and features of the tumor microenvironment .
Among the most promising of these emerging tools is the analysis of circulating tumor DNA (ctDNA). As Dr. Ben Ho Park, director of the Vanderbilt-Ingram Cancer Center, explains, all cells in the body secrete cell-free DNA into the bloodstream. In a cancer patient, a portion of this cell-free DNA comes from dying tumor cells.
This is ctDNA . A simple blood draw, often referred to as a liquid biopsy, can capture these genetic fragments. This technology allows oncologists to detect the presence of cancer at a molecular level, long before a tumor would be visible on a scan or cause physical symptoms .
Early Warning: The Power of Detection Before Clinical Relapse
The first, a long-term study published in JCO Precision Oncology, monitored 156 patients with primary breast cancer for up to 12 years after surgery and chemotherapy . Using Natera’s personalized Signatera test, researchers were able to detect ctDNA prior to clinical or radiologic relapse in 88.2% of patients who ultimately experienced a recurrence. Remarkably, the test predicted relapse up to 38 months in advance of it being detectable by conventional imaging, with a median lead time of 10.5 months .
The prognostic power of ctDNA was further validated by the LIBERATE study, results of which were published in ESMO Open in 2025. This study evaluated Guardant Health’s tissue-free MRD test, Guardant Reveal, in patients with early-stage breast cancer.
The findings were striking: the test demonstrated 100% sensitivity for distant recurrence in patients with ER+/HER2- breast cancer, the most common subtype, and a 100% positive predictive value for relapse .
The detection of ctDNA after surgery was significantly prognostic for event-free survival, providing a median lead time of 152 days (approximately five months) ahead of clinical recurrence .
This data signifies a paradigm shift. Traditionally, post-treatment surveillance has relied on periodic imaging scans and physical exams, which can only detect cancer once it has grown to a measurable size. ctDNA testing, by contrast, can identify molecular residual disease (MRD)—the tiny, undetectable deposits of cancer cells that may be the seeds of future metastasis.
As the authors of the EBLIS study concluded, “Serial postoperative ctDNA analysis has strong prognostic value and allows for earlier detection of recurrence than by scans in many patients” .
This “potential window” between a molecular and a clinical recurrence could be critical for designing trials of early therapeutic interventions, potentially improving outcomes before the cancer has a chance to establish itself fully .
Peace of Mind: A Patient’s Perspective on Living with ctDNA
Beyond the raw data and clinical implications, the true value of this biomarker breakthrough is perhaps best understood through the eyes of those it is designed to help. For survivors, the fear of recurrence is not an abstract concept but a daily psychological burden. Chloë Crampton, a stage II triple-negative breast cancer survivor diagnosed at the age of 32, eloquently articulates this reality.
In a blog post for Living Beyond Breast Cancer, she describes the “PTSD” that comes with survivorship, where “every pain or twinge makes you think, ‘Is it back?’” .
For Chloë, ctDNA testing has been a lifeline. After advocating for herself and requesting the Signatera test from her oncologist, she began a routine of testing every three months .
This regular rhythm has provided something that scans and doctor visits alone could not: consistent, tangible reassurance. “Hearing ‘you’re okay’ every three months helps calm my fears,” she shares. “I actually like the three-month cadence.
It’s a reminder that I’m still okay” .
The emotional impact cannot be overstated. Chloë describes how the testing has helped her begin to rebuild trust in her body—a trust she felt was shattered by her cancer diagnosis.
“I’ll never fully trust my body—it felt like it betrayed me—but I’ve started to believe in it again,” she says. “It’s helped me heal, move on, and feel hope for the future” .
Even when she experienced a scare during an MRI, a subsequent negative ctDNA test provided a calming influence amidst the stress . This perspective highlights a crucial dimension of precision medicine: its ability to address not only the physical disease but also the profound psychological wounds it leaves in its wake. While the medical community is still determining the best course of action following a positive ctDNA result—as there are not yet standardized guidelines for early-stage breast cancer—for many patients, the information itself is empowering . As Chloë puts it, “knowledge is power” .
Expanding the Biomarker Frontier: Beyond ctDNA
While ctDNA is currently at the forefront of the biomarker revolution, it is part of a much broader wave of innovation aimed at improving early detection and monitoring. Researchers are exploring a diverse array of bodily fluids and molecular signals to find the next generation of non-invasive tests.
One fascinating area of research is salivary metabolomics. A 2025 study published in Frontiers in Oncology investigated whether the metabolic compounds in saliva could serve as biomarkers for breast cancer. By analyzing saliva samples from breast cancer patients and healthy controls, researchers identified 101 different metabolites. Two compounds in particular, 2-aminonicotinic acid and theobromine (a compound also found in chocolate and coffee), showed promising diagnostic potential, with area-under-the-curve (AUC) scores of 0.81 and 0.75, respectively . This suggests that a simple saliva test could one day become a tool for early, non-invasive screening.
Another avenue of exploration involves combining traditional protein markers to create more powerful diagnostic tools. A study from Egypt investigated the serum levels of the tumor protein p53 (TP53), which is often mutated and stabilized in cancer cells. Researchers found that combining TP53 levels with the established marker CA125 into a single index, termed the “PCA-Index,” significantly improved early breast cancer detection. This index achieved an impressive AUC of 0.96 for early-stage tumors, with high sensitivity and specificity .
This “combinatorial analysis” approach, which integrates multiple biomarkers, is increasingly seen as a way to overcome the limitations of single-marker tests .
Furthermore, the scientific understanding of what constitutes a biomarker is expanding beyond the cancer cell itself. The tumor microenvironment—including immune cell infiltration and stromal composition—is now recognized as a critical source of prognostic information . Markers of immune response, for instance, are becoming increasingly important for guiding the use of immunotherapy in subtypes like triple-negative breast cancer .
Hope for the Future: The Road Ahead
The integration of these biomarker breakthroughs into routine clinical practice is still a work in progress. As the editors of the Living Beyond Breast Cancer blog note, at the time of publication, there are no actionable guidelines for a positive ctDNA result for people with early-stage breast cancer . Researchers themselves caution that while the technology is powerful, “properly controlled randomized studies will be needed to determine” how best to use this early warning to guide treatment decisions . There is a risk of over-treatment or anxiety from false positives, which have been observed, particularly in patients with HR+ disease .
Nevertheless, the trajectory is unmistakably positive. The technology is advancing rapidly, with companies like Natera and Guardant Health continuously publishing evidence that bolsters the case for broader adoption . The FDA has even granted “breakthrough device designation” to some of these tests, signaling their potential to offer a substantial improvement over existing alternatives .
The hope for the future is that this technology will democratize peace of mind and enable a more proactive, rather than reactive, approach to cancer care. Imagine a world where the end of treatment is not the beginning of endless waiting, but the start of a monitored journey where patients and their doctors are equipped with the tools to see—at a molecular level—that the coast is clear, or to intervene at the very first sign of trouble.
As Chloë Crampton’s story illustrates, the value of these tests transcends the laboratory. “Being part of the early group that helped bring ctDNA testing into wider use was powerful,” she reflects. “That feels amazing. But for me personally, the biggest impact has been emotional” .
The biomarker breakthrough in breast cancer is, therefore, a dual triumph. It is a triumph of science, offering a window into the disease that was unimaginable a generation ago
But it is also a triumph of the human spirit, providing survivors with a tool that helps quiet their deepest fears and empowers them to look toward the future with hope.
The journey from a static snapshot of a tumor to the dynamic, real-time monitoring of a patient’s molecular status is redefining what it means to be a survivor. It is transforming the landscape from one of uncertainty to one of informed, proactive, and hopeful living.
FAQs: Biomarkers for Breast Cancer Recurrence
1. How early can biomarkers detect breast cancer recurrence?
Biomarkers such as circulating tumor DNA (ctDNA) can detect microscopic cancer activity 6–10 months earlier than imaging scans or physical symptoms. This early signal allows doctors to start treatment sooner, when it is most effective.
2. Are biomarker tests a replacement for mammograms or scans?
No. Biomarkers are a powerful complement, not a replacement. They provide molecular-level insights that imaging cannot detect early, while scans confirm the location and size of a recurrence. Together, they offer a more complete monitoring strategy.
3. Is biomarker testing safe and non-invasive?
Yes. Most biomarker tests are done using a simple blood sample (liquid biopsy). They are safe, minimally invasive, and can be repeated regularly without the risks associated with frequent scans or biopsies.
References
- Biese, A. (2024, October 14). Circulating Tumor DNA May Predict Postsurgical Breast Cancer Relapse. CURE.
- Crampton, C. (2025, November 20). Finding peace in the waiting: Chloë Crampton on living with ctDNA testing. Living Beyond Breast Cancer.
- Decoding Breast Cancer: Emerging Molecular Biomarkers and Novel Therapeutic Targets for Precision Medicine. (2025). International Journal of Molecular Sciences, 27(1), 138.
- Jiang, X., et al. (2025). Identification of potential biomarkers for breast cancer based on salivary metabolomics. Frontiers in Oncology, 15, 1655213.
- Królewska-Daszczyńska, P., et al. (2025). The assessment of breast cancer biomarkers in diagnosis, prognosis and treatment monitoring: integrated analysis. Journal of Cancer Research and Clinical Oncology, 151(8), 233.
- Guardant Health. (2025, June 9). New Study Published in ESMO Open Highlights Guardant Reveal’s Performance in Detecting Minimal Residual Disease in Patients with Early-Stage Breast Cancer [Press release].
- Crampton, C. (2023, March 30). Circulating tumor DNA tests for peace of mind: Chloë Crampton. Living Beyond Breast Cancer.
- Sahin, A. A., et al. (2025). Emerging molecular Therapeutic targets in breast Cancer: Pathologic identification and clinical implications. Human Pathology, 105881.
- El-Far, M., et al. (2025). PCA-Index: The combination of serum levels of p53 and CA125 for breast cancer early detection. Laboratory Medicine, lmaf032.
- Natera, Inc. (2024, May 3). New Natera Publication Bolsters Evidence for Extended Surveillance with Signatera™ in Breast Cancer [Press release]. BioSpace.
Dr. Mohammed Abdul Azeem Siddiqui, MBBS, M.Tech (Biomedical Engineering – VIT, Vellore)
Registered Medical Practitioner – Reg. No. 39739
Physician • Clinical Engineer • Preventive Diagnostics Specialist
Dr. Mohammed Abdul Azeem Siddiqui is a physician–engineer with over 30 years of dedicated clinical and biomedical engineering experience, committed to transforming modern healthcare from late-stage disease treatment to early detection, preventive intelligence, and affordable medical care.
He holds an MBBS degree in Medicine and an M.Tech in Biomedical Engineering from VIT University, Vellore, equipping him with rare dual expertise in clinical medicine, laboratory diagnostics, and medical device engineering. This allows him to translate complex laboratory data into precise, actionable preventive strategies.
Clinical Mission
Dr. Siddiqui’s professional mission centers on three core pillars:
Early Disease Detection
Identifying hidden biomarker abnormalities that signal chronic disease years before symptoms appear — reducing complications, hospitalizations, and long-term disability.
Preventive Healthcare
Guiding individuals and families toward longer, healthier lives through structured screenings, lifestyle intervention frameworks, and predictive diagnostic interpretation.
Affordable Evidence-Based Treatment
Delivering cost-effective, scientifically validated care accessible to people from all socioeconomic backgrounds.
Clinical & Technical Expertise
Across three decades of continuous practice, Dr. Siddiqui has worked extensively with:
Advanced laboratory analyzers and automation platforms
• Cardiac, metabolic, renal, hepatic, endocrine, and inflammatory biomarker systems
• Preventive screening and early organ damage detection frameworks
• Clinical escalation pathways and diagnostic decision-support models
• Medical device validation, calibration, compliance, and patient safety standards
He is recognized for identifying subclinical biomarker shifts that predict cardiovascular disease, diabetes, fatty liver, kidney disease, autoimmune inflammation, neurodegeneration, and accelerated biological aging long before conventional diagnosis.
Role at IntelliNewz
At IntelliNewz, Dr. Siddiqui serves as Founder, Chief Medical Editor, and Lead Clinical Validator. Every article published is:
Evidence-based
• Clinically verified
• Technology-grounded
• Free from commercial bias
• Designed for real-world patient and physician decision-making
Through his writing, Dr. Siddiqui shares practical health intelligence, early warning signs, and preventive strategies that readers can trust — grounded in decades of frontline medical practice.
Contact:
powerofprevention@outlook.com
📌 Disclaimer: The content on IntelliNewz is intended for educational purposes only and does not replace personalized medical consultation. For individual health concerns, please consult your physician.