This document provides an overview of the safety and potential cancer risks associated with urogynaecological surgical implants used in women. These implants, primarily surgical mesh, are utilized to treat conditions like stress urinary incontinence (SUI) and pelvic organ prolapse (POP).

Shoutout:
“Millions of women rely on urogynaecological implants such as vaginal mesh and bladder slings for urinary incontinence and pelvic organ prolapse. Current evidence shows that these implants are not linked to cancer, but women should remain aware of possible complications and seek regular follow-up. Knowledge and early care empower women to make informed decisions about their pelvic health.”
— Dr. Mohammed Abdul Azeem Siddiqui | Contact
While they can offer significant benefits in improving quality of life, concerns have been raised regarding complications and, less frequently, potential links to cancer. This document aims to summarize the current understanding of these risks, highlighting the importance of informed consent, careful patient selection, and ongoing surveillance.
Urogynaecological surgical implants, most commonly surgical mesh, are medical devices used to provide support to weakened or damaged tissues in the pelvic floor. They are primarily used in the surgical management of:
Stress Urinary Incontinence (SUI): Involuntary urine leakage during activities like coughing, sneezing, or exercise. Mesh slings are often used to support the urethra.
Pelvic Organ Prolapse (POP): Descent of pelvic organs (bladder, uterus, rectum) into the vagina. Mesh can be used to reinforce weakened pelvic floor tissues.
Millions of women worldwide have received urogynecological implants such as vaginal mesh or bladder slings to treat bladder leakage and pelvic organ prolapse.
Over the years, alarming stories online have raised fears about cancer risk from surgical mesh.
So what is true—and what is not?

CRISPR Liquid Biopsy – Women’s Cancers
Learn how CRISPR liquid biopsies are revolutionizing detection of breast, ovarian, endometrial, and cervical cancers for women.
This article explains, in clear and simple language:
- Whether vaginal mesh can cause cancer
- What science actually shows
- The real risks women should know
- Warning signs you should never ignore
- What to ask your doctor
What Are Urogynecological Implants?
These are medical devices placed during pelvic surgery to support weakened tissues.
They are used to treat:
- Stress Urinary Incontinence (SUI)—bladder leakage when coughing, sneezing, or exercising
- Pelvic Organ Prolapse (POP) – when the bladder, uterus, or rectum drops into the vagina
Common Types
- Transvaginal mesh
- Mid-urethral slings (bladder slings)
- Pelvic floor support implants
Most are made of polypropylene plastic, designed to stay in the body permanently.
Do These Implants Cause Cancer?
🔬 The scientific answer:
No strong clinical evidence shows that vaginal mesh or bladder slings cause cancer.
Large medical studies involving thousands of women found:
- No higher cancer rates compared to women without implants
- No proven link to bladder, vaginal, or pelvic cancers
- Extremely rare cancer cases with no clear cause from mesh
Why did cancer fears begin?
- Animal studies showed tumors around foreign materials
- Social media and lawsuits spread fear
- Rare case reports created confusion
But human studies do not support a cancer connection.
Can Mesh Still Cause Serious Health Problems?
Yes. While cancer is not proven, other long-term complications are real.
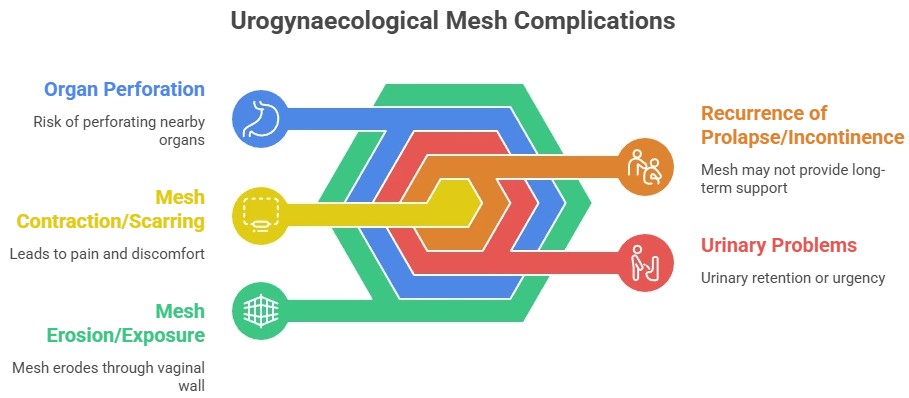
Common Vaginal Mesh Complications
- Chronic pelvic or vaginal pain
- Pain during sex
- Mesh erosion (mesh pokes through tissue)
- Recurrent infections
- Urinary or bowel problems
- Scar tissue and nerve pain
- Need for repeat surgery
These complications—not cancer—are why some mesh products were removed from the market.
Warning Signs You Should Never Ignore
If you have had pelvic mesh surgery, see a doctor urgently if you experience:
- Vaginal bleeding after menopause
- Persistent pelvic pain
- Foul-smelling discharge
- Burning during urination
- Pain during sex
- Repeated urinary infections
- Feeling a hard or sharp object inside the vagina
⚠️ These symptoms do not mean cancer, but they require medical care.
FDA Position (United States)
- Transvaginal mesh for prolapse is no longer sold
- Bladder slings are still allowed with strict monitoring
- No FDA warning links mesh to cancer
- Long-term safety tracking continues
Should Mesh Be Removed?
Not always.
If you feel well and have no symptoms, removal is not recommended just due to fear.
Removal is considered if you have:
- Chronic pain
- Erosion
- Infection
- Organ damage
Always see a urogynecologist experienced in mesh care.
What to Ask Before Surgery
- Are non-mesh options available?
- What type of implant will be used?
- What are the long-term risks?
- How will I be monitored?
- What symptoms should I report?
Other countries (UK, EU, Canada, Australia) follow similar rules.
Informed consent protects you.
How to Know If You Have Vaginal Mesh
Many women had bladder or prolapse surgery years ago and were never told the word “mesh.” If you are unsure, here’s how to confirm:
Medical devices used to support weakened pelvic tissues in women, often for bladder leakage or pelvic organ prolapse.
Yes. Most are referred to as surgical mesh.
No. Large human studies have found no proven link between these implants and cancer.
Bladder, vaginal, cervical, and pelvic cancers.
No. Cancer rates are similar in women with and without mesh.
From media reports, lawsuits, and confusion with animal studies.
Most are made from polypropylene plastic.
Yes, it is widely used in medical devices.
No health authority classifies pelvic mesh as cancer-causing.
Because of complications like pain and erosion, not cancer.
Yes, for stress urinary incontinence under strict guidelines.
Pain, infection, erosion, and painful intercourse.
Yes, localized inflammation can occur.
No, inflammation and cancer are different.
No. Preventive removal is not recommended for cancer fear.
If she has pain, bleeding, discharge, or urinary problems.
Yes, which is why evaluation is important.
No, it does not interfere with Pap smears or imaging.
Yes, some follow women for over 10–15 years.
Yes, under varying regulations.
No global study shows increased cancer risk.
They aim to reduce complications, not cancer risk.
Yes, some women experience pain during intercourse.
Yes, many live symptom-free.
There is no proven cancer link, but informed care is essential.
Signs you may have mesh
- You had surgery for bladder leakage (SUI) or pelvic organ prolapse (POP)
- You were told you received a “sling,” “tape,” or “support implant”
- Your surgery occurred between 2000 and 2019 (peak mesh use period)
How to confirm
- Request your operative report from the hospital or surgeon
- Ask for the implant or device record
- Get a pelvic exam by a urogynecologist
- In some cases, ultrasound or MRI is used
What Is Vaginal Mesh?
Vaginal mesh (also called pelvic mesh or bladder sling) is a surgical implant used to treat:
- Stress urinary incontinence (SUI)
- Pelvic organ prolapse (POP)
It is usually made of polypropylene plastic and is intended to provide long-term tissue support.
Mesh Erosion Symptoms (Most Common Signs)
Mesh erosion (also called mesh exposure or extrusion) happens when the implant wears through vaginal tissue or nearby organs.
Common Symptoms
- Persistent pelvic or vaginal pain
- Pain during sex (dyspareunia)
- Vaginal bleeding or discharge
- Recurrent urinary tract infections
- Burning during urination
- Feeling a hard or sharp area inside the vagina
- Partner feeling mesh during intercourse
- Difficulty sitting or walking
- Foul vaginal odor
⚠️ These symptoms do not mean cancer, but they require medical evaluation.
Bladder Sling Side Effects Years Later
Many women develop symptoms 5–15 years after surgery, including:
- Chronic pelvic pain
- Urinary urgency or leakage returning
- Pain with intercourse
- Nerve pain in hips, legs, or groin
- Recurrent infections
- Scar tissue and tightening
- Difficulty emptying bladder
These delayed effects happen because tissue changes over time around the mesh.
Vaginal Mesh Complications (Non-Cancer Risks)
While cancer is not linked, mesh can cause:
- Chronic inflammation
- Organ perforation
- Mesh contraction
- Adhesions and scarring
- Bowel or bladder injury
- Emotional distress and reduced quality of life
Many women require revision or removal surgery.
How to Know If You Have Vaginal Mesh
If you had surgery for bladder leakage or prolapse and are unsure:
Steps to confirm
- Ask your surgeon or hospital for your operative report
- Request your implant record or device card
- Get a pelvic exam by a urogynecologist
- Imaging (ultrasound or MRI) may help in complex cases
Pelvic Mesh Lawsuit – Eligibility Guide
You may qualify for legal review if you:
✔ Had transvaginal mesh or bladder sling surgery
✔ Developed pain, erosion, infection, or sexual dysfunction
✔ Required mesh revision or removal
✔ Have permanent complications
✔ Can document medical treatment
What lawsuits focus on
- Failure to warn patients
- Design defects
- Long-term complications
- Quality-of-life harm
⚠️ Lawsuits do not prove cancer risk — they address injury and device failure.
When to Seek Medical Help Immediately
- Sudden pelvic pain
- Bleeding not related to periods
- Recurrent infections
- Pain during sex
- Urinary retention
- Feeling mesh in the vagina
Key Takeaway
There is no proven link between pelvic mesh and cancer, but serious long-term complications do occur in some women. Early evaluation and specialist care can prevent worsening damage.
Bladder Sling Side Effects Years Later
Many women develop symptoms 5–15 years after mesh surgery due to tissue scarring, nerve irritation, or mesh erosion.
Common delayed symptoms
- Chronic pelvic or vaginal pain
- Pain during sex
- Burning or frequent urination
- Recurrent UTIs
- Groin, hip, or leg nerve pain
- Difficulty emptying the bladder
- Return of bladder leakage
- Tightness or pulling sensations
- Fatigue and emotional distress
These symptoms often worsen without treatment.
Pelvic Mesh Eligibility Guide
You may be eligible for medical or legal review if you:
✔ Had bladder sling or prolapse mesh surgery
✔ Have chronic pelvic pain, infections, or sexual dysfunction
✔ Were diagnosed with mesh erosion, scarring, or organ injury
✔ Required revision or removal surgery
✔ Have lasting physical or emotional harm
What cases focus on
- Failure to warn patients
- Defective mesh design
- Long-term injury
- Reduced quality of life
⚠️ Lawsuits focus on device complications, not cancer.
When to Seek Medical Help
- Vaginal bleeding or discharge
- Pain during sex
- Recurrent UTIs
- Feeling mesh inside the vagina
- Sudden pelvic pain
Easy Summary
- ✅ No proven link between mesh and cancer
- ⚠️ Complications can occur and must be monitored
- 🩺 Early care prevents worsening damage
- 💬 Clear communication saves lives
Final Thought
Fear spreads faster than facts.
Based on current global medical research:
Urogynaecological implants are not linked to cancer—but they must be used carefully, monitored closely, and discussed honestly.
Knowledge empowers women to make safe, confident decisions.
1. Do urogynaecological implants or vaginal mesh cause cancer?
No. Current medical research shows no proven link between vaginal mesh, bladder slings, or urogynaecological implants and cancer in humans.
2. What are the most common complications of vaginal mesh?
The real risks are not cancer, but:
- Chronic pelvic or vaginal pain
- Pain during sex
- Mesh erosion into nearby tissue
- Recurrent infections
- Urinary or bowel problems
3. How can I tell if I have vaginal mesh and it’s causing problems?
You may have mesh if you had surgery for bladder leakage or prolapse.
See a doctor if you notice bleeding, pain, discharge, UTIs, or feel mesh inside the vagina.
A pelvic exam or imaging (ultrasound/MRI) can confirm it.
4. Should vaginal mesh be removed?
Not always. If you feel well, removal is not recommended.
Removal is considered only when there is pain, erosion, infection, or organ damage—and should be done by an experienced urogynecologist.
Medical Disclaimer:
This article is for educational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare provider regarding any questions or concerns about urogynaecological implants, vaginal mesh, bladder slings, or any medical condition. Do not ignore professional medical advice or delay seeking it because of information found on this site.
References / Citations
- FDA. (2019). Urogynecologic surgical mesh: FDA actions and safety information. U.S. Food and Drug Administration. Retrieved from https://www.fda.gov/medical-devices/urogynecologic-surgical-mesh
- Maher, C., Feiner, B., Baessler, K., & Christmann-Schmid, C. (2013). Surgical management of pelvic organ prolapse in women. Cochrane Database of Systematic Reviews, 4, CD004014. https://doi.org/10.1002/14651858.CD004014.pub5
- Abbott, S., et al. (2014). Long-term outcomes of midurethral slings for stress urinary incontinence. International Urogynecology Journal, 25(12), 1685–1692. https://doi.org/10.1007/s00192-014-2454-3
- FDA. (2011). Urogynecologic surgical mesh: UPDATE on transvaginal mesh for pelvic organ prolapse. U.S. Food and Drug Administration. Retrieved from https://www.fda.gov/medical-devices/urogynecologic-surgical-mesh/update-urogynecologic-surgical-mesh
- Clemons, J. L., & Webster, G. D. (2015). Complications of synthetic midurethral slings. Current Urology Reports, 16(6), 42. https://doi.org/10.1007/s11934-015-0517-2
- Scott, K. G., & Karram, M. M. (2012). Mesh complications in gynecologic surgery: Incidence, management, and clinical outcomes. International Urogynecology Journal, 23(Suppl 1), S5–S12. https://doi.org/10.1007/s00192-012-1703-4
- Anger, J., et al. (2017). Safety and efficacy of surgical mesh in treating female stress urinary incontinence and pelvic organ prolapse: Systematic review and meta-analysis. American Journal of Obstetrics and Gynecology, 216(2), 132–144. https://doi.org/10.1016/j.ajog.2016.10.015
- International Urogynecological Association (IUGA). (2020). Statement on the use of mesh in pelvic organ prolapse and stress urinary incontinence. Retrieved from https://www.iuga.org
- Faltin, D. L., et al. (2013). Risk of malignancy after pelvic reconstructive surgery using synthetic mesh: A population-based cohort study. BJOG: An International Journal of Obstetrics & Gynaecology, 120(7), 841–847. https://doi.org/10.1111/1471-0528.12161
- Maher, C., Feiner, B., Baessler, K., & Christmann-Schmid, C. (2016). Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database of Systematic Reviews, 2, CD012079. https://doi.org/10.1002/14651858.CD012079.pub2
Dr. Mohammed Abdul Azeem Siddiqui, MBBS, M.Tech (Biomedical Engineering – VIT, Vellore)
Registered Medical Practitioner – Reg. No. 39739
Physician • Clinical Engineer • Preventive Diagnostics Specialist
Dr. Mohammed Abdul Azeem Siddiqui is a physician–engineer with over 30 years of dedicated clinical and biomedical engineering experience, committed to transforming modern healthcare from late-stage disease treatment to early detection, preventive intelligence, and affordable medical care.
He holds an MBBS degree in Medicine and an M.Tech in Biomedical Engineering from VIT University, Vellore, equipping him with rare dual expertise in clinical medicine, laboratory diagnostics, and medical device engineering. This allows him to translate complex laboratory data into precise, actionable preventive strategies.
Clinical Mission
Dr. Siddiqui’s professional mission centers on three core pillars:
Early Disease Detection
Identifying hidden biomarker abnormalities that signal chronic disease years before symptoms appear — reducing complications, hospitalizations, and long-term disability.
Preventive Healthcare
Guiding individuals and families toward longer, healthier lives through structured screenings, lifestyle intervention frameworks, and predictive diagnostic interpretation.
Affordable Evidence-Based Treatment
Delivering cost-effective, scientifically validated care accessible to people from all socioeconomic backgrounds.
Clinical & Technical Expertise
Across three decades of continuous practice, Dr. Siddiqui has worked extensively with:
Advanced laboratory analyzers and automation platforms
• Cardiac, metabolic, renal, hepatic, endocrine, and inflammatory biomarker systems
• Preventive screening and early organ damage detection frameworks
• Clinical escalation pathways and diagnostic decision-support models
• Medical device validation, calibration, compliance, and patient safety standards
He is recognized for identifying subclinical biomarker shifts that predict cardiovascular disease, diabetes, fatty liver, kidney disease, autoimmune inflammation, neurodegeneration, and accelerated biological aging long before conventional diagnosis.
Role at IntelliNewz
At IntelliNewz, Dr. Siddiqui serves as Founder, Chief Medical Editor, and Lead Clinical Validator. Every article published is:
Evidence-based
• Clinically verified
• Technology-grounded
• Free from commercial bias
• Designed for real-world patient and physician decision-making
Through his writing, Dr. Siddiqui shares practical health intelligence, early warning signs, and preventive strategies that readers can trust — grounded in decades of frontline medical practice.
Contact:
powerofprevention@outlook.com
📌 Disclaimer: The content on IntelliNewz is intended for educational purposes only and does not replace personalized medical consultation. For individual health concerns, please consult your physician.



